We assisted with Commissioning, Qualification, and Validation (CQV) support for a new facility expansion to support our client’s rapid growth. Learn how we delivered in spite of challenges.
Biotechnology
SERVICES
Project Management
Commissioning Qualification & Validation
Engineering Analysis
Report Writing
Development and Revision of SOPs
SKILLS
Validation
Quality Assurance
The Challenge
Oxford Global Resources and a large Biotechnology Contract Manufacturing Organization have a long-standing and successful partnership. We were brought in as a primary partner to assist with Commissioning, Qualification, and Validation (CQV) support for a new facility expansion to support our client’s rapid growth. This would involve a new construction build-out and a mix of transferred and new equipment for the space. However, they lacked a robust talent pool to support their initiative. Based on our relationship, knowledge, expertise, and experience, the client determined we would be the best partner to work with them.
The Solution
Before this engagement, the Oxford team worked to create a specific project plan that would account for their rapid growth both now and in the future and create efficiencies. We provided a group of 11 expert consultants, including a CQV Engineer, Validation Engineer, Method Validation Engineer, and Project Manager, Process Scientist, and Supplier Auditors. These individuals worked with the project management team to keep track of action items and activities in the project plan. In addition, these experts:
- Wrote CQV protocols
- Executed CQV activities
- Summarized data results
- Supported engineering analyses
- Wrote CQV reports and assisted with tech transfer and change control activities
- Developed or revised SOPs
In addition to their CQV expertise, our consultants worked with several special types of equipment on this project, including:
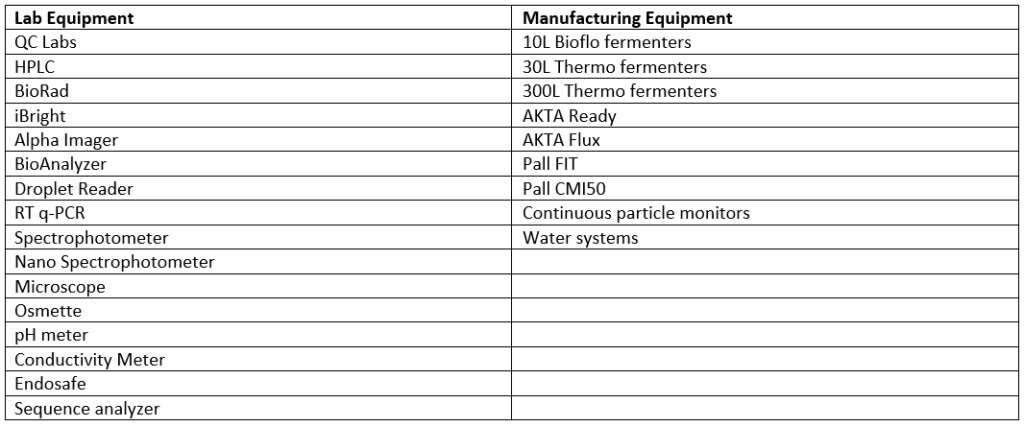
Table of Lab and Manufacturing Equipment
The Result
Despite the client’s remote location and uncontrollable delays caused by the weather, the Oxford Consultants helped complete the facility expansion within a year, including all validation activities. Because we developed a positive working relationship with the client team, they called upon us to assist them in several other areas of their operations, including Computer System Validation, Data Integrity Transformation, Quality Control initiatives, Lab Validation efforts, Test Method Validation, technical operations improvements, manufacturing operations optimization, Quality Assurance/Batch Record Review, Supplier Audits, and GAP Assessments.
.svg.png)


