In 2020, it is anticipated that there will be 1,806,590 new cases of cancer reported with an estimated number of over 606,502 deaths.1 In order to effectively collect and analyze this data and provide better patient outcomes, organizations partner with Certified Tumor Registrars.
What are the Responsibilities of a Certified Tumor Registrar?
A Certified Tumor Registrar (CTR) is an individual who mines data and captures information from health records of cancer patients, prepares reports and performs data analysis on the data collected, and monitors patients’ treatment and outcomes. The information collected includes but is not limited to:
- Demographic information
- Patient history
- Diagnostic findings
- Cancer information
- Cancer treatment
- Follow-up information
This data allows organizations to evaluate cancer incidence and survival rates, monitor and advance cancer treatments, and improve cancer prevention and screening programs. A CTR may also be responsible for attending, coordinating, and preparing minutes and reports at Cancer Conference or Tumor Board sessions.
Certification
A CTR is the certification offered by the National Cancer Registrars Association (NCRA) which sets the standard in the field of cancer registry.3 A CTR should have thorough knowledge of anatomy and physiology, and will possess such skills as organization, critical thinking and problem-solving, time management, communication, presentation, and data analysis. These individuals should be detail-oriented, investigative, disciplined, and meticulous.4 To receive the certification, a CTR must pass an exam offered by the NCRA and usually possess at least an Associate’s Degree or Certificate in Cancer Registry Management.5 In 2015, the Commission on Cancer (COC) made it a requirement that all cancer case abstracting be performed by a CTR.2
Cancer Registries
There are many different types of cancer registries. The three basic types are hospital registries, central (population-based) registries, and special purpose registries. A hospital registry may choose to become accredited by the COC or the National Cancer Institute (NCI), but is not required to do so. Central registries have more specific requirements for operations as they are regulated by the North American Association of Central Center Registries (NAACCR), who sets the gold standard in the field or the NCI Surveillance Epidemiology and End Results (SEER). The first hospital registry was established at Yale-New Haven Hospital is 1926, while the first central registry was established in Connecticut in 1935. A National Registry did not exist until 1992 when Congress established a National Program of Cancer Registries.6
Data Collection Pathway
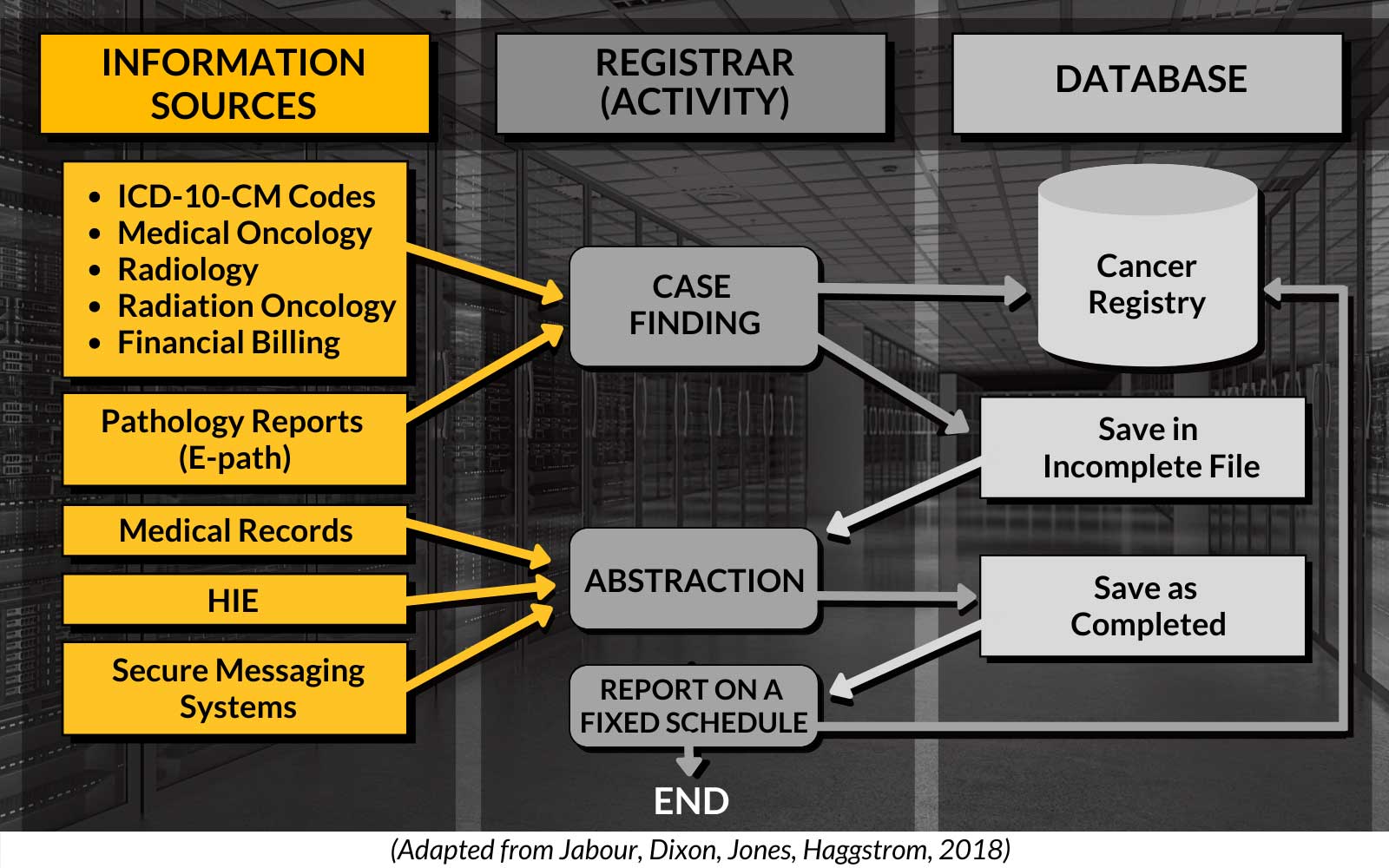
Importance of Cancer Registries
Cancer is the second leading cause of death in the United States and costs about $107 billion dollars a year in healthcare expenses and loss of productivity from illness and death.7 Reducing the impact of cancer requires data to learn more about cancer causes, treatments and follow-ups. This enables clinicians to control the disease and improve patient care. Many people, including physicians, researchers, and legislators are working together to accomplish this mission; however, they rely on the data mined by CTRs to make decisions, optimize available funds, and implement screening and treatment programs.7
Challenges Facing Cancer Registries
There are many challenges that a cancer registry faces, including limited awareness, competing priorities, lack of IT support, and the burden of manual reporting.8 Manual reporting includes the amount of time and money spent to identify cases, treatment, and the correct managing physician; and complete and submit required forms. In addition to these challenges, other difficulties may be faced if treatments are provided outside of the hospital facility. For example, oncologists might mistrust hospital treatment reporting requests due to the belief that these are efforts to identify additional patients to recruit.8
What Does this Mean for Your Organization?
When making a determination of how best to approach your CTR strategy, consider the following questions:
- Do you have an existing tumor registry program? Are you a part of a population-based program? If so, which one?
- Is your current program accredited or non-accredited?
- What type of reporting (NAACCR, SEER, State) do you use?
- What duties do your registrars perform (case-finding, abstracting, Tumor Board or tumor conference, follow-ups)?
In order to effectively reduce backlog abstracting or prepare for an accreditation survey, it is critical to work with certified CTRs. Oxford’s certified CTRs have experience with Tumor Board and Cancer Conference presentations; case-finding, abstracting, and follow-ups; accredited and non-accredited programs; and NCDB, SEER, and various state submissions. When considering a CTR to partner with, ask them the following questions:
- What type of reporting have they performed (NAACCR, SEER, State)?
- In addition to abstracting, what duties have they performed?
With the right partnership, organizations can effectively evaluate the data in their cancer patients’ health records and make a definitive impact on the treatment of this disease. A certified CTR may be the key to effectively and meaningfully improving patient care and reducing the impact of cancer in the United States.
References
- NIH (n.d.) Cancer stat facts: Cancer of any site. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer.gov/statfacts/html/all.html
- American College of Surgeons (2019). Presentation of Major Changes/New Standards. https://www.facs.org/-/media/files/qualityprograms/cancer/presentations/2019_coc_ed_summit/2019_coc_ed_killackey_major_changes.ashx
- NCRA (2020d). Council on certification. Retrieved from https://www.ncra-usa.org/ctr
- NCRA (2020b). Become a cancer registrar: Skills and Strengths. https://www.ncra-usa.org/About/Become-a-Cancer-Registrar/Skills-Strengths
- NCRA (2020a). Become a cancer registrar: Paths to become a cancer registrar https://www.ncra-usa.org/About/Become-a-Cancer-Registrar/Paths-to-Become-a-Cancer-Registrar
- NCRA (2020c). Cancer registry profession. https://www.ncra-usa.org/About/Cancer-Registry-Profession
- SEER Training Modules (n.d.) Importance of cancer registry. U. S. National Institutes of Health, National Cancer Institute. https://training.seer.cancer.gov/registration/registry/importance.html
- Bickell, N. A., Wellner, J., Franco, R., Scheck-McAlearney, A. (2013). New accountability, new challenges: Improving treatment reporting to a tumor registry. Journal of Oncology Practice. https://ascopubs.org/doi/10.1200/JOP.2012.000843
- Jobour, A. M., Dixon, B. E., Jones, J. F., and Haggestrom, D. A. (2018, January 3). Toward timely data for cancer research: Assessment and reengineering of the caner reporting process. Journal of Medical Internet Research. https://cancer.jmir.org/2018/1/e4/
.svg.png)


